A new study on human forebrain cortical organoids, cell cultures mimicking the early development of this part of the brain, suggests that exposure to polystyrene microplastics alters gene expression and neural tissue patterning in these cells. Additionally, cells had shorter lifespans when exposed to this type of microplastics for a period of up to 30 days. The study was published in the Journal of Hazardous Materials.
The past century has seen a huge increase in the use of plastics. Current estimates are that worldwide plastic production increases by 300 million tons each year. Much of that plastic ends up as garbage either on land or in the seas. However, unlike many other materials, most types of plastic do not degrade into components that can be used by plants and animals. When exposed to elements and various environmental factors, plastics gradually break down into smaller and smaller pieces.
Eventually these pieces of plastic become very small, measuring a few millimeters and even less than that. Such pieces are called microplastics. Microplastic particles can easily be transported through air and water and become a part of biological systems. They get ingested by marine organisms. Humans can also drink them with water or eat them with food without noticing as these particles are very small.
A type of plastic most frequently observed in marine and land organisms is polystyrene. Polystyrene is used to make a wide range of products, including disposable containers, packaging materials, insulation, and foam products like styrofoam. As polystyrene products are very often single use or disposable, polystyrene easily ends up in the environment.
Study author Timothy Hua and his colleagues wanted to examine the effects of exposure to polystyrene microplastics on the development of the human brain. They note that the human central nervous system is highly vulnerable to environmental toxins during development. Due to this, there is a large risk that exposure to microplastics could lead to cellular damage and increased inflammation of the brain.
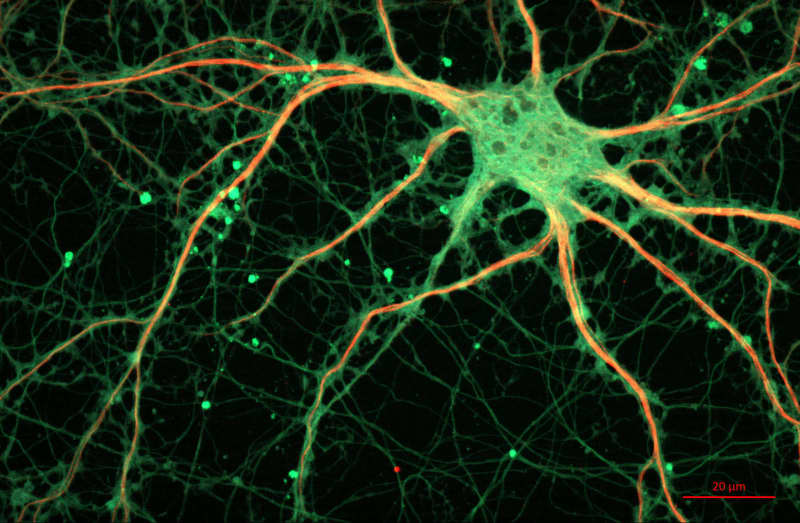
To achieve this, they used human induced pluripotent stem cells-derived brain organoids. Human induced pluripotent stem cell-derived brain organoids are miniature three-dimensional structures cultivated in a laboratory from reprogrammed human cells. These organoids mimic some aspects of the human brain’s structure and function. Because of this, they are a valuable tool for studying brain development, effects of diseases and drugs in manners that would not be possible on human brains due to ethical considerations.
Stem cells are undifferentiated cells that can develop into various specialized cell types in the body. In this study,the researchers induced stem cells (iPSK3 cells) to differentiate into cells of the brain cortex. In this way, the researchers formed cell cultures – organoids that underwent a process of differentiation similar to the one happening during embryonic development and formation of the brain cortex.
The microplastics used in the experiment were sterilized polystyrene microplastic particles 1 or 10 micrometers in diameter. On day 4 since the start of cell differentiation, the researchers divided the organoids into several groups. Depending on the group, they exposed them to solutions containing different concentrations of microplastic particles and different particle sizes.
The researchers used 5, 50 or 100 milligrams per milliliter concentrations and either 1 micrometer or 10 micrometer plastic particles. They also had a control group of organoids that were not exposed to microplastics. They replaced the microplastic solution every 2-3 days either up to day 10, for short-term exposure, or up to day 30, for examining long-term exposure.
The results showed that microplastics do not accumulate in the cell culture. Ten micrometer particles tended to degrade into smaller ones, less than 2 micrometers in diameter, after more than 15 days in the cell culture. This finding is important, because the 10 micrometer particles are roughly the same size as cells and were expected to stay in the space between cells (extracellular space) of the organoid. Their breaking up into smaller sizes allowed them to be uptaken by the cells, the same way 1 micrometer particles would be.
During the first 6 days of microplastic treatment, between days 4 and 10 of cell differentiation, exposure to microplastics resulted in normal differentiation of neurons and increased cell division. The cells retained normal capacity to produce SOD2 (manganese superoxide dismutase), an enzyme important for protecting cells from oxidative stress (damage to cells due to processes of oxidation).
On the molecular level, short-term exposure to microplastics (up to 10 days) led to changes in expressions of a number of genes. These changes in gene expression differed for microplastic size and concentrations. Cell cultures exposed to microplastics for up to 30 days also showed multiple changes to gene expressions.
Estimation of the shares of live and dead cells in the cell cultures showed that the percentage of live cells was lower in organoids exposed to microplastics compared to those without such exposure. Percentage of live cells was the lowest in organoids exposed to concentrations of 50 microgram per milliliter of particles regardless of whether the particles were 1 or 10 micrometers. Exposure to 10 micrometer particles in concentrations of 100 micrograms per milliliter resulted in the same share of live cells as in untreated organoids.
“The results of quantitative and qualitative measurements in this study suggest that the size and concentration-dependent effects of polystyrene microplastics on developing forebrain cerebral spheroids may induce high intercellular/intracellular stress and adversely affect cortical layer differentiation. While short-term microplastic exposure promotes cell proliferation and neural progenitor gene expression, the long-term microplastic exposure reduces cell viability and downregulates more mature neuronal marker and cortical layer VI marker expression,” the study authors concluded.
The study makes an important contribution to the scientific understanding of effects of microplastics exposure on brain development. However, it also has limitations that need to be taken into account. Notably, the study was conducted on cell cultures immersed in a medium to which researchers could introduce microplastics at will. This is profoundly different from the environment in which the development of human brain takes place. Additionally, the cell differentiation process carried out experimentally on stem cells might not be identical to the natural process of brain development in human embryos.
The study, “Microplastics exposure affects neural development of human pluripotent stem cell-derived cortical spheroids”, was authored by Timothy Hua, Sonia Kiran, Yan Li, and Qing-Xiang Amy Sang.
