
FDA and Reagan-Udall Foundation are hosting a virtual public meeting on psychedelic research titled Advancing Psychedelic Clinical Study Design, which aims to explore empirical approaches to address issues in drug development.
The U.S. Food and Drug Administration (FDA) together with the Reagan-Udall Foundation for the FDA are hosting a virtual public meeting on psychedelic research, Jan. 31 to Feb. 1.
Titled "Advancing Psychedelic Clinical Study Design," the event aims to explore "empiric approaches to address key issues in psychedelic drug development and research."
In June 2023, the federal agency issued its first draft guidance providing general considerations to industry sponsors developing psychedelic drugs for the treatment of medical conditions. The document was commented on, among others, by specialized lawyer Mason Marks.
While the guidance highlights some considerations for designing psychedelic clinical trials to optimize the interpretation of results, several questions remain regarding the most appropriate way to address such challenges.
Also Read: State-Regulated Psychedelics ‘On A Collision Course’ With FDA, Says Harvard Law Expert
The meeting will provide an overview of the FDA's guidance, explore perspectives and current research, as well as have scientists share their experience working with psychedelics in FDA-authorized clinical studies and drug development.
The Heat Index In Research, Where All Starts
Although the scientific subworld of psychedelics represents a comparatively small percentage when considering all people working or participating in the psychedelics industry, understanding what goes on and how things are moving at the research level is key, as it may very well stir the direction of the whole industry and, most importantly, affect whether and how patients will eventually be treated.
The conversation kicked off with remarks byDr. Patrizia Cavazzoni, director of the FDA's Center for Drug Evaluation and Research.
This was followed by a presentation by Dr. Tiffany R. Farchione, director of the federal agency's Psychiatry Division within the Neuroscience Office, who shared unpublished internal analysis data on the number of psychedelic Investigational New Drug (IND) applications received since 2000.
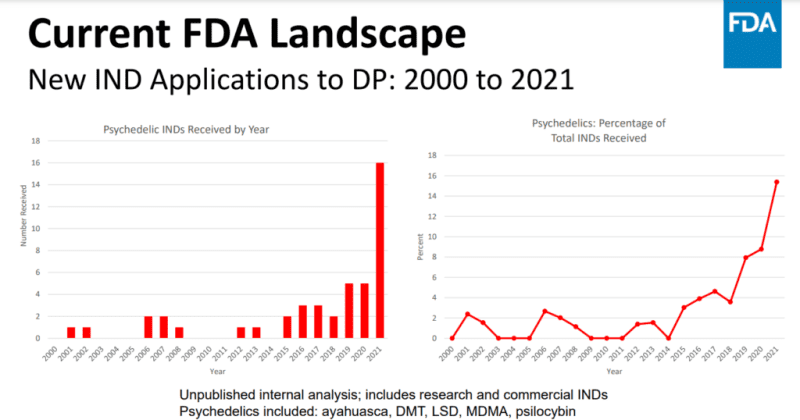
As informed by Psychedelic Alpha, this analysis only includes Investigational New Drug (IND) applications related to psychiatry, so submissions concerning psychedelics for conditions like substance use disorders are not included.
The Jan. 31 sessions (four total) delved into discussing the challenges in selecting control conditions to create blinding for psychedelic studies to reduce bias and determine whether changes in results measures are actually due to the psychedelic.
It also touched on issues concerning drug dosing, such as dose response, single versus repeat dosing, and microdosing with MDMA, psilocybin and LSD among the most advanced psychedelic compounds in science as of today.
The speakers’ list includes Mind Medicine Inc.’s (NASDAQ: MNMD) Robert Barrow, NYU Langone's Dr. Michael Bogenschutz, Johnson & Johnson's (NYSE: JNJ) Dr. Carla Canuso, Usona Institute's Dr. Michael Davis and top officials from various FDA divisions.
The Feb. 1 sessions will have representatives from Johns Hopkins University, the University of California San Francisco, the FDA, the Veterans Administration, Harvard University, the University of Utah, Bar-Ilan University, the Federation of State Medical Boards and more. Topics to be covered: set and setting, overview of FDA regulatory authority and considerations for potential psychedelic use in the real world. Register here.
Photo: Benzinga edit with photo courtesy of Serrgey75 and fizkes on Shutterstock.
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.

