A baby girl who was born deaf can hear unaided for the first time, thanks to a breakthrough gene therapy she received at Addenbrooke’s Hospital.
Opal Sandy is the first patient treated in a global trial that doctors say could lead to the dawn of a new era for gene therapies in those with hearing loss.

They described the results as “spectacular”.
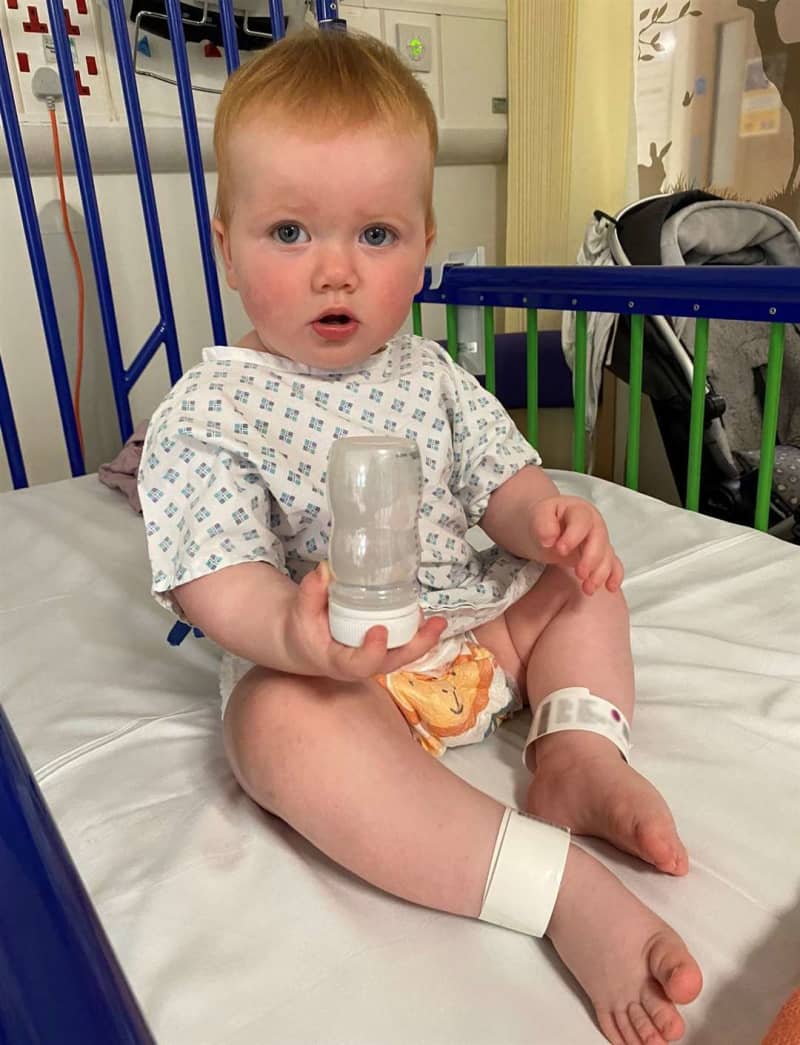
Opal is the first British patient in the world and became the youngest child to receive this type of treatment when she was given it at 11 months.
She was born completely deaf due to a rare genetic condition called auditory neuropathy that is caused by the disruption of nerve impulses travelling from the inner ear to the brain.
But within four weeks of receiving the gene therapy infusion to her right ear, Opal responded to loud clapping - even with the cochlear implant in her left ear switched off.
Opal’s mother, Jo Sandy, described the results as “mind-blowing” and said: “When she first turned, I couldn’t believe it. I thought it was a fluke or like a change in light or something that had caught her eye, but I repeated it a few times. I picked my phone up and texted James, and said ‘I think it’s working’. I was absolutely gobsmacked. I thought it was a fluke.”

But in the weeks that followed, clinicians noticed continuous improvement in Opal’s hearing and at 24 weeks confirmed that she has close to normal hearing levels for soft sounds, such as whispering, in her treated ear.
“We were so happy when the clinical team confirmed at 24 weeks that her hearing was also picking up softer sounds and speech. Everyone was so excited such amazing results had been achieved,” said Jo.
“The audiologist played back some of the sounds that she was responding to and they were ridiculously quiet sort of sounds that in the real world wouldn’t catch your attention during a conversation.
“Since February, we’ve noticed her sister (Nora) waking her up in the morning because she’s running around on the landing, or someone rings on the door so her nap’s cut short.
“She’s definitely responding more to sort of what we would call functional sounds rather than just sounds that we use to test her.
“We were told she had near normal hearing last time – I think they got responses at sort of 25 to 30 decibels.
“I think normal hearing is classed at 20 decibels, so she’s not far off. Before, she had no hearing whatsoever.”
The gene therapy was created by US biotech firm Regeneron.

Prof Manohar Bance, chief investigator of the trial, and an ear surgeon at Cambridge University Hospitals NHS Foundation Trust, which runs Addenbrooke’s, said: “These results are spectacular and better than I expected. Gene therapy has been the future of otology and audiology for many years and I’m so excited that it is now finally here. This is hopefully the start of a new era for gene therapies for the inner ear and many types of hearing loss.”
Auditory neuropathy can be caused by the variation of a single gene - OTOF - which produces a protein called otoferlin that is needed to allow the inner hair cells in the ear to communicate with the hearing nerve.
About 20,000 people across the UK, Germany, France, Spain and Italy are deaf due to a mutation in the OTOF gene.
Children with a variation in this gene often pass the standard newborn screening test because the hair cells are working, but they are not communicating with the nerve.
This means their hearing loss may not be detected until they are two or three years old, by which time it will have caused a noticeable delay in their speech development.
The CHORD trial, which was launched in May 2023, was designed to show whether gene therapy can provide hearing for children born with auditory neuropathy.
Prof Bance said: “We have a short time frame to intervene because of the rapid pace of brain development at this age. Delays in the diagnosis can also cause confusion for families as the many reasons for delayed speech and late intervention can impact a children’s development.”
Standard NHS genetic testing can detect mutations in the OTOF gene.
In Opal’s case, she was identified as being at risk as her older sister has the condition. A genetic test when she was three weeks old confirmed she had it too.
In the trial, Opal was given an infusion containing a harmless virus called AAV1, which delivered a working copy of the OTOF gene via an injection in the cochlea during surgery carried out under general anaesthesia.
While Opal, from Oxfordshire, was being given the gene therapy in right ear during the procedure, a cochlear implant was also fitted in her left ear.
James Sandy, Opal’s father said: “It was our ultimate goal for Opal to hear all the speech sounds. It’s already making a difference to our day-to-day lives, like at bath-time or swimming, when Opal can’t wear her cochlear implant.
“We feel so proud to have contributed to such pivotal findings, which will hopefully help other children like Opal and their families in the future.”
Opal’s 24-week results and other scientific data from the CHORD trial are being presented at the American Society of Gene and Cell Therapy (ASGC) in Baltimore, USA, this week.
Prof Bance said: “More than 60 years after the cochlear implant was first invented – the standard of care treatment for patients with OTOF related hearing loss – this trial shows gene therapy could provide a future alternative. It marks a new era in the treatment for deafness.
“It also supports the development of other gene therapies that may prove to make a difference in other genetic related hearing conditions, many of which are more common than auditory neuropathy.”
A second child has also received the gene therapy treatment at Cambridge University Hospitals, and positive results have recently been recorded, just six weeks after surgery.
Three phases of the CHORD trial are planned, with three children, including Opal, receiving a low dose of gene therapy in one ear only in the first.
Another three children are due to receive a high dose in one ear in phase two. And if that too proves safe, a third phase will look at gene therapy in both ears with the dose selected after ensuring the safety and effectiveness in the first two parts.
Up to 18 children from the UK, Spain and the US are being recruited to the trial, with follow-up appointments continuing for five years for enrolled patients to show how patients adapt to understand speech in the longer term.
Prof Bance said: “My entire life, gene therapy has been ‘five years away’, and I’ve been in practice about 30 years.
“So, for me, it was almost unreal that this moment had arrived. We’ve been hearing about this for so long, and there’s been so much work, decades of work - to finally see something that actually worked in humans, it was quite spectacular and a bit awe-inspiring really. It felt very special.”
Dr Richard Brown, consultant paediatrician at CUH, who is an investigator on the CHORD trial, said: “The development of genomic medicine and alternative treatments is vital for patients worldwide, and increasingly offers hope to children with previously incurable disorders.
“It is likely that in the long run such treatments require less follow-up so may prove to be an attractive option, including within the developing world. Follow-up appointments have shown effective results so far with no adverse reactions and it is exciting to see the results to date.
“Within the new planned Cambridge Children’s Hospital, we look forward to having a genomic centre of excellence which will support patients from across the region to access the testing they need, and the best treatment, at the right time.”
Martin McLean, senior policy advisor at the National Deaf Children’s Society, said:
“Many families will welcome these developments, and we look forward to learning about the long-term outcomes for the children treated. This trial will teach us more about the effectiveness of gene therapy in those cases where deafness has a specific genetic cause.
“We would like to emphasise that, with the right support from the start, deafness should never be a barrier to happiness or fulfilment. As a charity, we support families to make informed choices about medical technologies, so that they can give their deaf child the best possible start in life.”
In Cambridge, the trial is supported by NIHR Cambridge Clinical Research Facility and NIHR Cambridge Biomedical Research Centre.
Prof Stephen Powis, NHS England’s national medical director, said: “This trial will transform the life of 18-month-old Opal Sandy while offering hope to many others like her, and is another example of the NHS being a global leader in developing gene therapy for patients.
“The NHS has led the world in the development of a range of other innovations and therapies, and this is a very welcome addition to the work going on with life sciences organisations around England to expand the range of treatments available.”
