
Imagine sparkling diamonds, not forged over millions of years in the Earth’s fiery depths, but whipped up in a lab in just a quarter of an hour. This scenario just became a reality thanks to a groundbreaking new method developed by scientists in South Korea. Their research could shatter the traditional diamond market and usher in a new era of diamond production.
Growing diamonds
For decades, growing diamonds in a lab has relied on replicating the Earth’s mantle — a colossal undertaking requiring immense pressure and scorching temperatures to coax carbon to turn into synthetic diamonds. This method, known as high-pressure, high-temperature (HPHT) growth, is not only energy-intensive and time-consuming (weeks), but it also yields limited results. HPHT diamonds are capped at around a blueberry’s size, and the process struggles to produce anything larger.
The new technique developed by Dr. Rodney Ruoff and his team at the Institute for Basic Science in South Korea throws those limitations out the window. Instead of replicating the Earth’s fiery furnace, they’ve devised a surprisingly simple method that operates at sea-level pressure. Their secret lies in a specially designed chamber and the use of gallium, a metal that catalyzes the formation of graphene from methane.
Like diamond, graphene comprises pure carbon, however, their structures are worlds apart. Diamond is made of carbon atoms arranged in a very strong and rigid 3D network. Meanwhile, graphene is made of a single layer of carbon atoms arranged in a hexagonal lattice, almost like a chicken wire.
During their experiments, the researchers funneled superheated, carbon-rich methane gas through the special chamber. There, it encountered a crucible containing a unique mixture of gallium, nickel, iron, and a sprinkle of silicon.
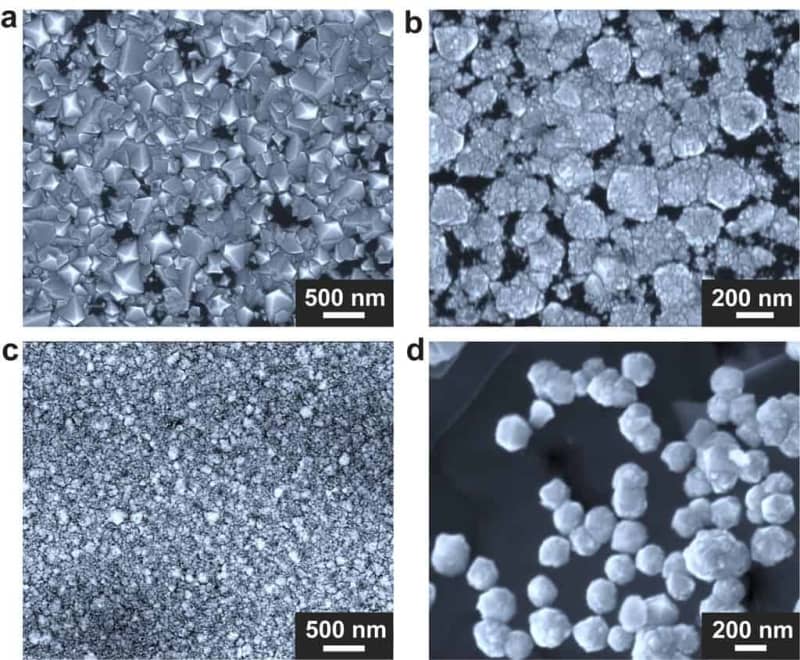
Within a mere 15 minutes, diamond deposits materialize on the crucible’s base. The resulting diamonds are almost pristine, largely made of carbon with a few silicon atoms of impurity. Although the exact mechanisms are still under investigation, the researchers believe a temperature drop within the chamber concentrates carbon, causing it to crystallize into diamonds. Silicon seems to play a crucial role in this process, potentially acting as a seed for diamond formation.
Impressive work in progress
“For over a decade I have been thinking about new ways to grow diamonds, as I thought it might be possible to achieve this in what might be unexpected (per ‘conventional’ thinking) ways,” Ruoff told Live Science.
There’s a catch, however. While the new method boasts incredible speed and simplicity, it comes at the cost of size. The diamonds produced are microscopic — too tiny to grace your finger or adorn a necklace. But there’s a silver lining. The low-pressure environment used in this technique has scientists optimistic about scaling up production. If they can figure out how to make these diamonds grow to a usable size, this could be a game changer for the industry.
So, what does the future hold for these minuscule diamonds? While they might not be dazzling on your finger anytime soon, their industrial potential is vast. In the future, new diamonds may be only a mere 15 minutes away.
“In about a year or two, the world might have a clearer picture of things like possible commercial impact,” Ruoff told Live Science.
The findings were reported in the journal Nature.
Was this helpful?
Thanks for your feedback!
This story originally appeared on ZME Science. Want to get smarter every day? Subscribe to our newsletter and stay ahead with the latest science news.
