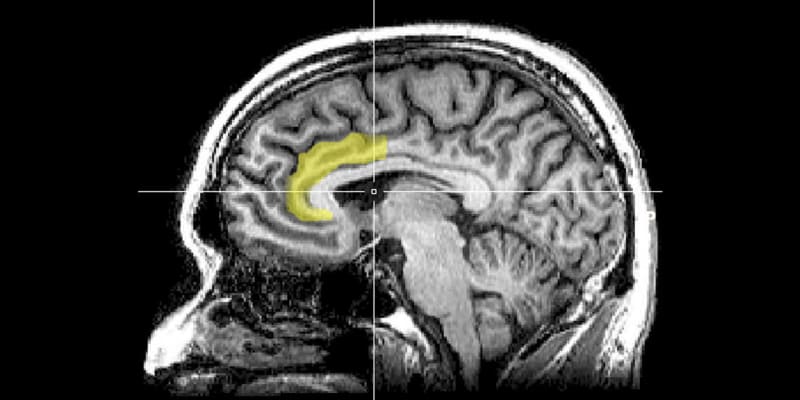
New research published in Translational Psychiatry sheds light on how ketamine, a drug known for its rapid antidepressant effects, specifically alters brain activity in people with treatment-resistant depression. This detailed investigation into the brain’s anterior cingulate cortex reveals that ketamine’s influence on different regions of this area correlates with notable improvements in depressive and anhedonic symptoms.
Ketamine, initially known as an anesthetic, has recently gained attention in the mental health community for its rapid antidepressant effects, particularly in individuals who do not respond to traditional treatments. The motivation behind this study arose from the need to understand how ketamine achieves these effects. Unlike most antidepressants that take weeks to show results, ketamine can lift mood within hours, making it a potential game-changer in acute depression care. However, its exact mechanisms of action in the brain have remained largely unexplored, especially regarding treatment-resistant depression, a condition where patients do not respond to standard antidepressant therapies.
“Ketamine is an antidepressant with several interesting properties: it acts quickly; it seems to work in treatment-resistant cases of depression; and it seems to be effective in treating symptoms which are normally difficult to treat, such as anhedonia (a lack of sensitivity to reward),” explained study author Laith Alexander, an academic clinical fellow at the Institute of Psychiatry at King’s College London.
“The anterior cingulate cortex (ACC) is implicated in depression and in how ketamine works, but no study had explored in detail how ketamine modulates activity in the ACC and whether this modulation is important in ketamine’s therapeutic effects. Furthermore, the ACC consists of different subregions – subgenual, perigenual and dorsal – and it isn’t clear which of these subregions are important.”
The study involved 50 participants, comprising 29 individuals with treatment-resistant depression and 21 healthy volunteers. These individuals were part of a larger, randomized clinical trial. Participants with depression had a history of not responding to at least one adequate antidepressant trial and exhibited significant symptoms as measured by the Montgomery-Åsberg Depression Rating Scale, a standard tool for assessing the severity of depressive episodes. Before undergoing brain scans, all participants with depression were medication-free for at least two weeks to ensure a clear assessment of ketamine’s effects.
The core of the study centered around a process called resting-state functional MRI (fMRI) imaging, a type of brain scan that measures brain activity by detecting changes associated with blood flow. This method is particularly useful for understanding how different brain regions communicate with each other when a person is not focusing on a specific task – hence the term ‘resting-state.’
In this double-blind study, participants received an intravenous infusion of either ketamine or a saline solution (placebo) during the first session and then received the opposite treatment two weeks later. Brain scans were conducted two days following each treatment. Additionally, the research team used several scales, including the Snaith–Hamilton Pleasure Scale and the Temporal Experience of Pleasure Scale, to measure levels of anhedonia.
The findings revealed that ketamine significantly improved depressive symptoms and the ability to anticipate pleasure compared to the placebo. Interestingly, ketamine’s effects varied across different areas within the anterior cingulate cortex – a part of the brain known to be involved in mood regulation.
For instance, changes in the functional connectivity between the perigenual anterior cingulate cortex and the right insula (a brain region involved in emotional processing) were associated with improvements in depression scores. In contrast, alterations in the connectivity of the subgenual anterior cingulate cortex to other brain regions, like the ventral striatum (associated with reward processing), correlated with reductions in anhedonia.
“When we found that changes in subgenual ACC connectivity correlated with improvements in anhedonia symptoms, this back-translated nicely to the preclinical animal literature,” Alexander told PsyPost. “Studies in primates have shown that changes in activity of this region can induce problems in reward processing, and that ketamine can act on this region to alleviate these deficits.”
The researchers also found that while the subgenual anterior cingulate cortex showed the most substantial changes in response to ketamine, the connectivity of the dorsal anterior cingulate cortex to the supramarginal gyrus (part of the somatosensory association cortex) was also modified. These nuanced findings highlight the complexity of depression and anhedonia and suggest that different brain regions may contribute to these conditions in diverse ways.
“The ACC seems to be a key region in ketamine’s antidepressant action, but specific subregions of the ACC seem to be more important in the relief of certain symptoms: for example, changes in pregenual ACC connectivity correlated with improvements in symptoms of depression whereas changes to subgenual ACC connectivity correlated with improvements in symptoms of anhedonia specifically,” Alexander explained. “By understanding how ketamine modulates connectivity of these different subregions, we may eventually be able to target specific treatments to certain groups of patients burdened by particular symptoms.”
Despite these promising results, the study is not without its limitations. The small sample size, particularly when exploring correlations between brain connectivity and symptom improvement, poses a challenge. “This was a small study, which needs to be replicated in a larger sample size to ensure the results are reliable,” Alexander said.
“This study was a good example of the importance of collaboration and open science,” the researcher added. :These data were analyzed from a double-blind randomized placebo-controlled trial carried out at the National Institute of Mental Health (NIMH). The collaboration between King’s College London and NIMH meant a hypothesis could be tested on pre-existing data.”
The study, “Preliminary evidence that ketamine alters anterior cingulate resting-state functional connectivity in depressed individuals“, was authored by Laith Alexander, Peter C. T. Hawkins, Jennifer W. Evans, Mitul A. Mehta, and Carlos A. Zarate Jr.
